Biogenic volatile organic compounds remove OH from the atmosphere through chemical reactions, which affects processes such as cloud formation. In a new study, Pfannerstill et al. reveal the important contributions of previously not-considered BVOCs species and underestimated OVOCs to the total OH reactivity.
Total OH reactivity
The tropical forests are Earth’s largest source of biogenic volatile organic compounds (BVOCs). These compounds are highly chemically reactive, which makes them very relevant to atmospheric processes. Once released to the atmosphere, BVOCs undergo oxidation reactions within seconds to days. BVOCs react mainly with OH radicals, which form during the daytime primarily from the ozone (O3) and water. They thus remove part of the OH from the atmosphere, making tropical forests an OH-sink. The abundance of OH in the atmosphere in turn affects processes such as cloud formation and the residence of greenhouse gases such as methane.
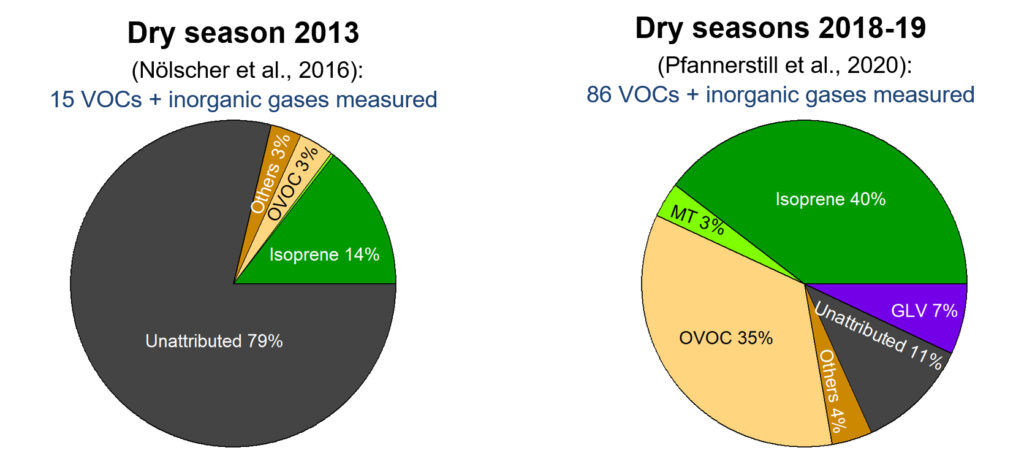
Despite the impact of BVOCs on large-scale atmospheric processes, most studies in tropical forests to date have been limited to a small number of known, abundant BVOC species. But they cannot explain the total OH-sink, and substantial fractions of up to 80% remained unaccounted for.
Now Eva Pfannerstill and her colleagues wanted to solve this problem and close the budget. During several field campaigns at ATTO, they measured the loss of OH molecules per second, which is called “Total OH reactivity”. At the same time, they measured BVOC emissions and ozone concentrations.
Finding the missing link
Due to this comprehensive approach, they were able to largely attribute all the OH reactivity to specific compounds. This includes a total of 83 different VOC species and 3 inorganic trace gases. Previously unaccounted for OH reactivity in the rainforest was due to a combination of things.
On the one hand, Pfannerstill and her co-authors found a number of primary BVOCs that were not included in previous studies, but that do contribute significantly to OH reactivity.
On the other hand, they found that oxygenated compounds (OVOCs) appear to play an important role. The team’s analysis reveals a contribution of OVOCs to the seasonal total OH reactivity of roughly 1/3. This is up to 5 times more than what scientists found in previous studies. An explanation for the large contribution of OVOCs to the OH sink is likely the meteorological conditions in the tropics. Intense solar irradiation, high humidity leading to high OH levels, and elevated temperatures cause fast photochemical oxidation processes.
In addition, they analyzed daily, season, interannual cycles of OH-reactivity at various heights above the forest canopy. Among others, they found that rainfall causes short-term spikes in total OH reactivity, which are followed by below-normal OH reactivity for several hours.
Pfannerstill et al. published all their results Open Access in “Total OH reactivity over the Amazon rainforest: variability with temperature, wind, rain, altitude, time of day, season, and an overall budget closure” in Atmos. Chem. Phys.
Similar articles
Direct measurements of OH radicals are rare and difficult to achieve. However, since they react with BVOCs, Ringsdorf et al. inferred them from isoprene measurements at ATTO. To do so, they applied a technique called ‘Dynamical Time Warping’ from the field of speech recognition. Akima Ringsdorf et al. published the study “Inferring the diurnal variability of OH radical concentrations over the Amazon from BVOC measurements” Open Access in Nature Scientific Reports.
Eliane Gomes Alves and her colleagues measured isoprene emissions at the ATTO 80-meter tower across three years to better understand how these emissions vary seasonally and under extreme climatic conditions like El Niño events. They also looked into which biological and environmental factors regulate the emission of isoprene to the atmosphere.
In a new study, Denis Leppla, Thorsten Hoffmann and their colleagues looked at pinic acid and its chiral forms. Pinic acid forms in the atmosphere through SAO formation from α-pinenes. The team wanted to find out how the chemical reactions in the atmosphere affect the chirality of its product pinic acid.
Bioaerosols influence the dynamics of the biosphere underneath. In a new study, Sylvia Mota de Oliveira and her colleagues used the ATTO site to collect air samples at 300 m above the forest. Then, they used DNA sequencing to analyze the biological components that were present and figure out what species of plant or fungi they belong to. One of the most striking new insights is the stark contrast between the species composition in the near-pristine Amazonian atmosphere compared to urban areas.
BVOC emissions in the Amazon have been studied for decades, but we still don’t fully understand when and under what conditions tree species or even individual trees emit more or fewer isoprenoids. To address this, Eliane Gomes Alves and her colleagues measured isoprenoid emission capacities of three Amazonian hyperdominant tree species.
Mosses and lichen appear to play a previously overlooked but important role in the atmospheric chemistry of tropical rainforests. A new study from Achim Edtbauer and colleagues shows that such cryptogams emit highly reactive and particle-forming compounds (BVOCs) that are important for air quality, climate, and ecosystem processes.
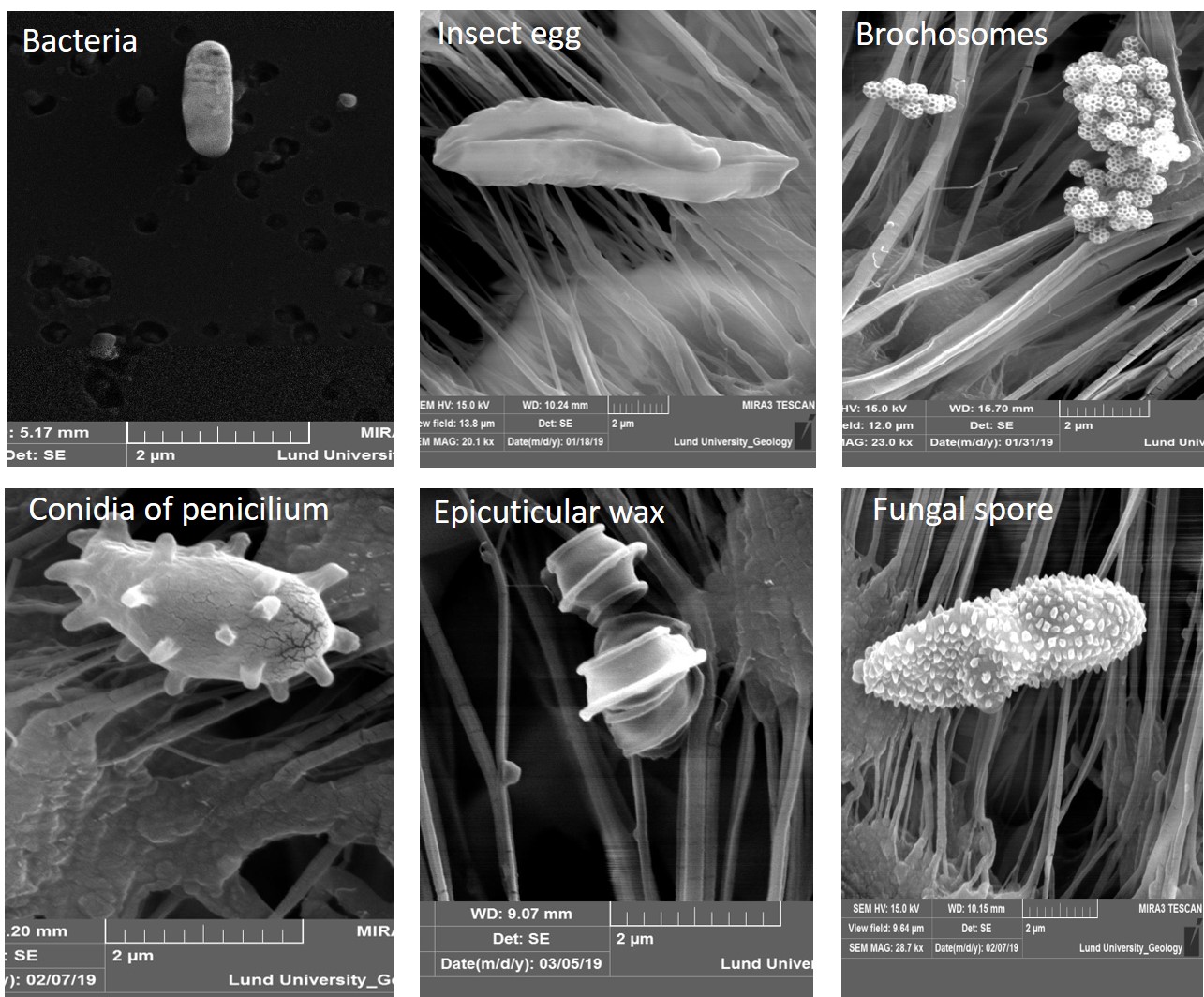
The Amazon rain forest plays a major role in global hydrological cycling. Biogenic aerosols, such as pollen, fungi, and spores likely influence the formation of clouds and precipitation. However, there are many different types of bioaerosols. The particles vary considerably in size, morphology, mixing state, as well as behavior like hygroscopicity (how much particles attract water) and metabolic activity. Therefore, it is likely that not only the amount of bioaerosols affects the hydrological cycle, but also the types of aerosols present.
Bioaerosols may act as cloud condensation nuclei and ice nuclei, thereby influencing the formation of clouds and precipitation. But so far there is less knowledge about the ice nucleation activity of each bioaerosol group and atmospheric models hitherto have not differentiated between them. Patade et al. created a new empirical parameterization for five groups of bioaerosols, based on analysis of the characteristics of bioaerosols at ATTO: fungal spores, bacteria, pollen, plant/animal/viral detritus, and algae. This makes it possible for any cloud model to access the role of an individual group of bioaerosols in altering cloud properties and precipitation formation.
Felipe Souza, Price Mathai and their co-authors published a new study analyzing the diverse bacterial population in the Amazonian atmosphere. The composition varied mainly with seasonal changes in temperature, relative humidity, and precipitation. On the other hand, they did not detect significant differences between the ground and canopy levels. They also identified bacterial species that participate in the nitrogen cycle.
Nora Zannoni and her colleagues measured BVOC emissions at the ATTO tall tower in several heights. Specifically, they looked at one particular BVOC called α-pinene. They found that chiral BOVs at ATTO are neither equally abundant nor is the ratio of the two forms constant over time, season, or height. Surprisingly, they also discovered that termites might be a previously unknown source for BVOCs.